2021-08-24-da Cara Therapeutics va uning biznes hamkori Vifor Pharma o‘zining birinchi sinfdagi kappa opioid retseptorlari agonisti difelikefalin (KORSUVA™) surunkali buyrak kasalligi (CKD) bemorlarni davolash uchun FDA tomonidan tasdiqlanganligini e’lon qildi (ijobiy o‘rtacha/og‘ir gemodit bilan davolash boshlanadi), 2022 yil 1-chorak. Cara va Vifor Amerika Qo'shma Shtatlarida KORSUVA™ ni tijoratlashtirish bo'yicha eksklyuziv litsenziya shartnomasini imzoladilar va KORSUVA™ ni Fresenius Medical kompaniyasiga sotishga kelishib oldilar. Ularning orasida Cara va Viforning har biri Fresenius Medical'dan tashqari savdo daromadlarida 60% va 40% foyda ulushiga ega; Ularning har biri Fresenius Medical kompaniyasining savdo tushumida 50% foyda ulushiga ega.
CKD bilan bog'liq qichishish (CKD-aP) dializdan o'tayotgan KKD bemorlarida yuqori chastota va intensivlik bilan yuzaga keladigan umumiy qichishishdir. Qichishish dializ bilan og'rigan bemorlarning taxminan 60-70 foizida uchraydi, ulardan 30-40 foizi hayot sifatiga jiddiy ta'sir ko'rsatadigan (masalan, yomon uyqu sifati) va depressiya bilan bog'liq bo'lgan o'rtacha/qattiq qichimaga ega. Ilgari CKD bilan bog'liq qichishish uchun samarali davolash yo'q va Difelikefalinni tasdiqlash katta tibbiy ehtiyoj bo'shlig'ini bartaraf etishga yordam beradi. Ushbu tasdiqlash NDA topshirishdagi ikkita muhim bosqich III klinik sinovlariga asoslanadi: AQShda va butun dunyo bo'ylab KALM-1 va KALM-2 sinovlarining ijobiy ma'lumotlari va KORSUVA ™ yaxshi muhosaba qilinganligini ko'rsatadigan 32 ta qo'shimcha klinik tadqiqotlarning qo'llab-quvvatlovchi ma'lumotlari.
Yaqinda Yaponiyada difelikefalinni klinik tadqiq qilishdan yaxshi xabar keldi: 2022-1-10, Cara uning hamkorlari Maruishi Pharma va Kissey Pharma gemodializ bilan og'rigan bemorlarda qichishishni davolash uchun Yaponiyada difelikefalin in'ektsiyasi qo'llanilishini tasdiqlaganini e'lon qildi. III bosqich klinik sinovlar Birlamchi yakuniy nuqtaga erishildi. 178 bemor 6 haftalik difelikefalin yoki platsebo qabul qildi va 52 haftalik ochiq yorliqli kengaytmali tadqiqotda ishtirok etdi. Birlamchi yakuniy nuqta (qichishishning raqamli shkalasi ko'rsatkichining o'zgarishi) va ikkilamchi yakuniy nuqta (Shiratori og'irlik shkalasi bo'yicha qichishish ko'rsatkichining o'zgarishi) platsebo guruhiga nisbatan difelikefalin guruhida boshlang'ich darajadan sezilarli darajada yaxshilandi va yaxshi muhosaba qilindi.
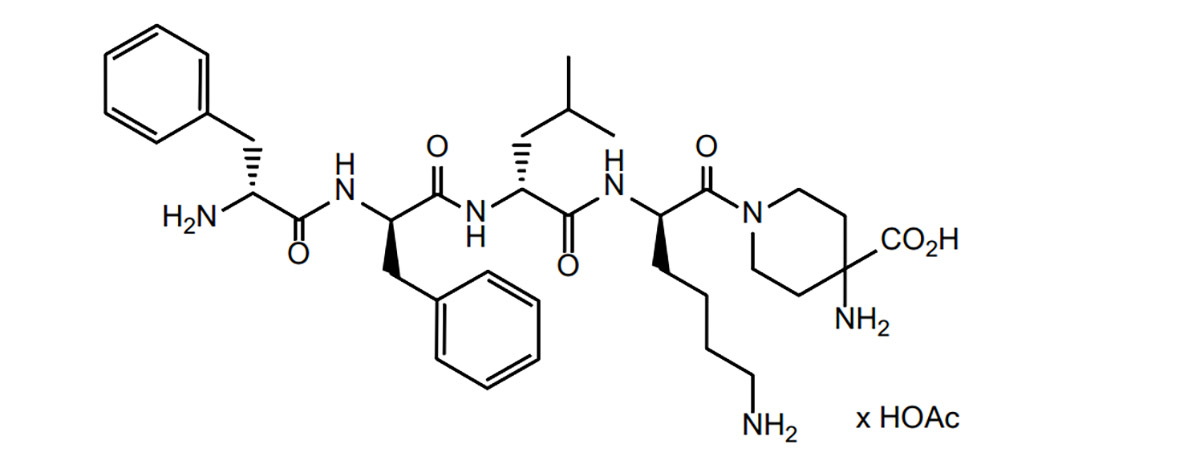
Difelikefalin opioid peptidlar sinfidir. Bunga asoslanib, Peptid tadqiqot instituti opioid peptidlari bo'yicha adabiyotlarni o'rganib chiqdi va opioid peptidlarining dori vositalarini ishlab chiqishdagi qiyinchiliklari va strategiyalarini, shuningdek, dori vositalarini rivojlantirishning hozirgi holatini umumlashtirdi.
Xabar vaqti: 2022 yil 17-fevral